Symple Surgical is an exciting start-up Medical Device company focused on developing novel therapeutic technologies through controlled Microwave Ablation. Our technologies are thoughtfully designed to be familiar and easy to use by the operator while providing automated control, feedback, and safety for the patient.
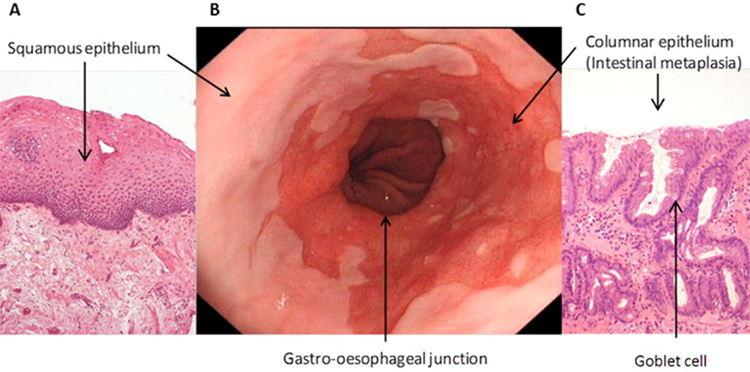
Symple Surgical’s first product offering is the DirectAblate GRIZZLYTM Microwave Ablation SystemTM. Through the ground-breaking therapy of directed microwave ablation of pre-cancerous Barrett’s cells, we are focused on helping millions of patients worldwide living with Barrett’s Esophagus and the subsequent advancement to esophageal adenocarcinoma (EAC). While multiple clinical studies have demonstrated that radiofrequency ablation for treatment of Barrett’s Esophagus is incompletely effective in 25% of patients studied, the novel utilization of a superior microwave ablation mechanism will overcome these difficult to treat patients. This superior mechanism includes more uniform heating, superior automation and control over energy deployment regions, and an antenna that can both emit and receive signals. Thermal ablation has proven to be a safe and effective way to treat Barrett’s Esophagus and EAC utilizing a short one-time minimally invasive gastroenterology procedure.
Due to the unique characteristics of Microwave Energy, wide-ranging applications for this technology cross-over into the fields of Interventional Cardiology, Electrophysiology, Nephrology, Pulmonology and Vascular Intervention. Symple Surgical has established an exciting pipeline of products targeting growing markets in minimally invasive medicine.
Symple Surgical, Inc. is a privately-held company with engineering and product development offices in Sacramento, California and Flagstaff, Arizona. We are comprised of a highly experienced team of Medical Device professionals with global experience covering research and development (R&D) through product commercialization. Company founder and serial entrepreneur, Dan Kasprzyk, has a strong track record with several medical device companies from inception through exit. We are backed by a world class Scientific Advisory Board of clinicians, Mayo Clinic and researches dedicated to developing therapeutic microwave technology for superior treatment of life-threatening chronic conditions.
|
|
Our Technology |

|
Circumferential Ablation | Preserved Arterial Surface | Automated Ablation Activation
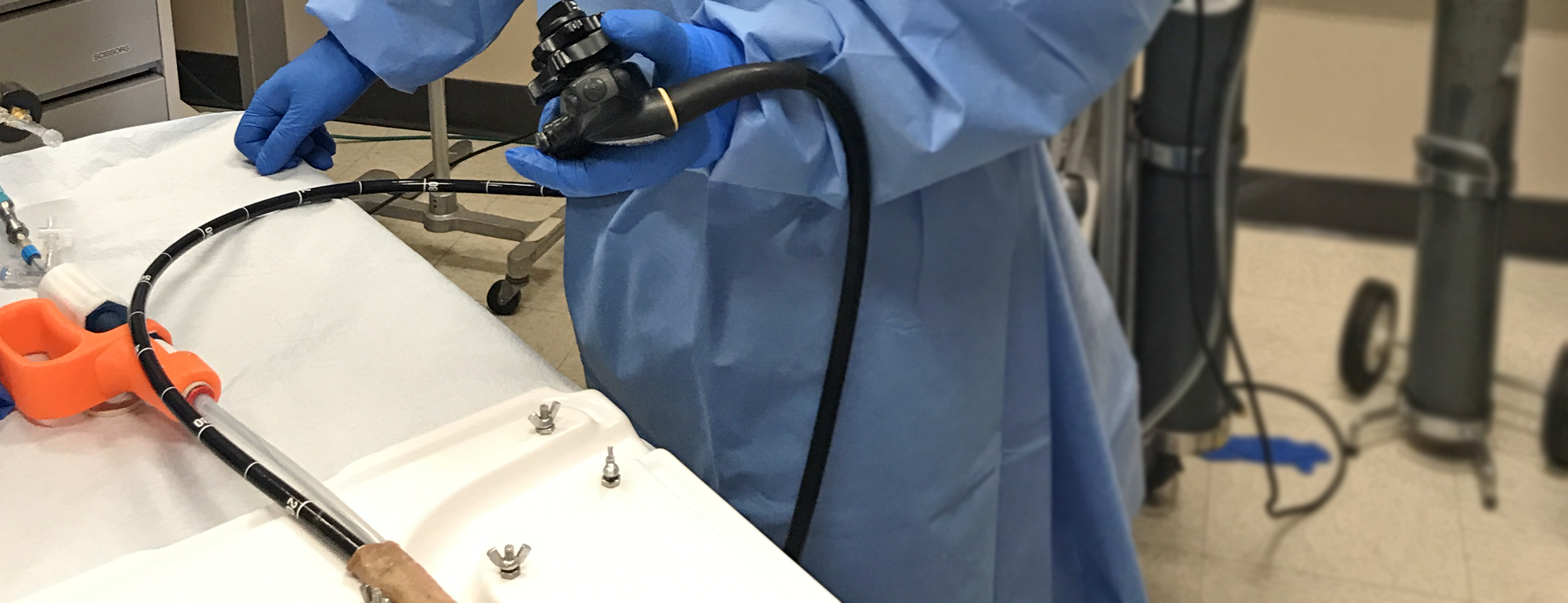
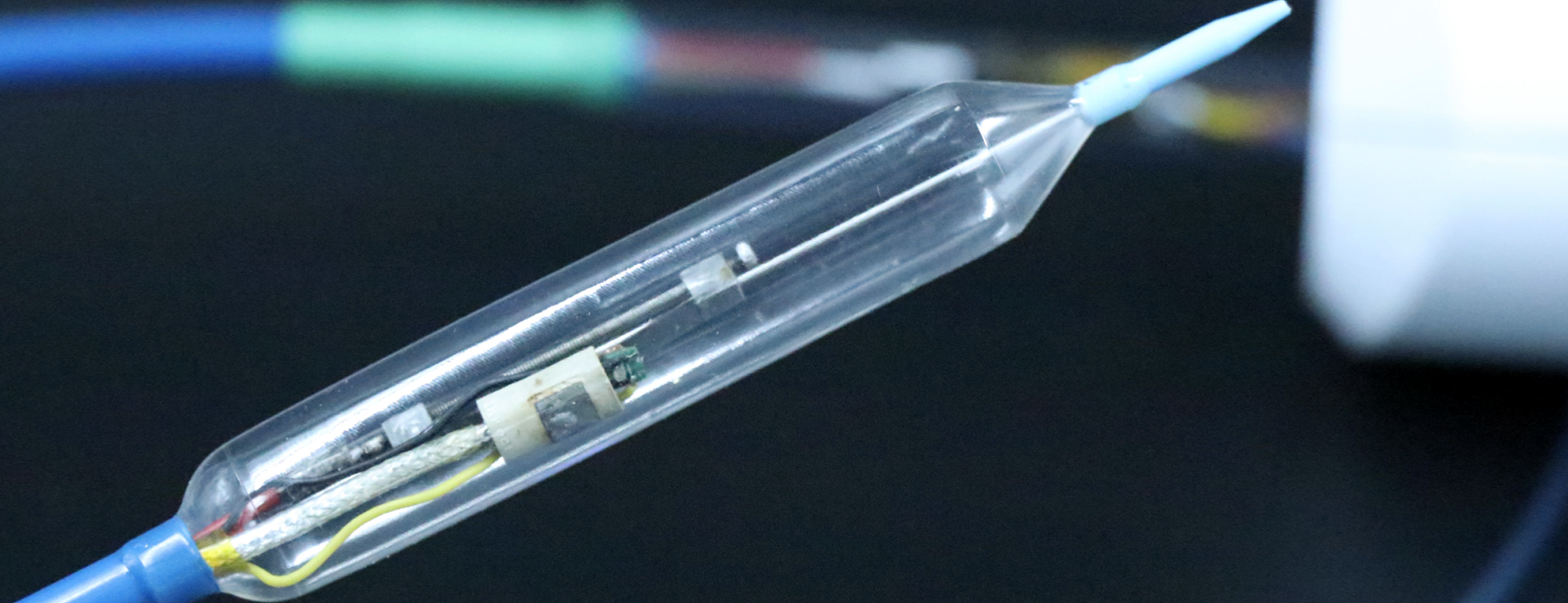
The Symple Surgical GRIZZLYTM microwave ablation catheter system is a beside-the-scope, over-the-wire, moveable antennae, compliant urethane balloon system. The catheter is a fully automated system that can create complete circumferential and spot ablations from a single device up to 40mm in length. A compliant urethane balloon provides the ability to target esophagus diameters between 18-35mm. Initial product offering will be a beside-the-scope catheter with .035 guidewire compatibility. At the heart of the GRIZZLYTM catheter is a proprietary microwave antenna which paired with the 50W, 18GHz microwave generator delivers precise and uniform microwave energy to the target ablation zone.
A secondary product is the DirectAblate Renal Denervation SystemTM which is a proprietary self-centering internally integrated microwave antenna, real-time surface temperature monitoring, and active arterial wall cooling for safety. A 100 Watt / 2.45 GHz microwave generator with integrated control system provides precise control and data acquisition. DirectAblate Renal Denervation SystemTM has superior catheter and energy delivery technology that effectively delivers a single 90 second circumferential non-contact ablation per renal artery.
| Validated Thermal Simulation |
 |
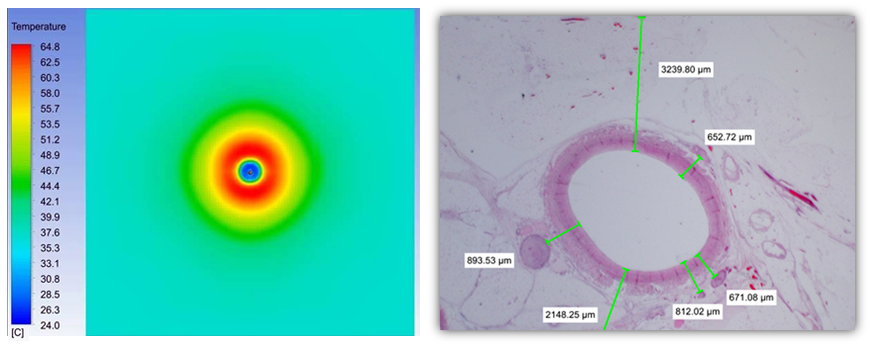
Through comprehensive thermal simulation and laboratory testing, Symple Surgical has optimized the DirectAblate Renal Denervation SystemTM to deliver therapeutic microwave energy to the site of the Sympathetic Nerves. Figure 1 shows Computer Generated Thermal Simulation data of the DirectAblate Renal Denervation SystemTM. Figure 2 is histological sample of an in vivo study performed with the DirectAblate Renal Denervation SystemTM. The treatment zone is nearly identical in size and shape to the computer based modeling as identified with NBT viability staining (blue/purple coloring is preserved tissue; translucent/white is treated tissue; rings are 1mm spacing).
| directed Renal Denervation (dRDN) |
 |
The Symple Surgical microwave device is demonstrated within a protein albumin gel highlighting the “doughnut-shape” circumferential ablation zone created. While penetrating 6mm deep in to the protein gel, the test demonstrates the thermal protection of the vessel wall and the 1mm ring of the medial layer. Past the 6mm ablation zone there is a substantial drop-off in temperature, thereby limiting damage to the non-target perivascular structures.

|
|
Our Team |
 |
Our Vision
To assemble a talented team focused on improving patient health through the development of novel Microwave Therapies while enriching our Employees’ lives. Foster a culture of innovation resulting in rapid and concise decision making with the ultimate goal of inventing and commercializing superior products for the treatment of chronic conditions.
| Founding Team |
 |
Two proven medical device entrepreneurs, Daniel J. Kasprzyk and Randy Preston originally founded Symple Surgical in 2012.
Mr. Preston has over 15 years’ experience in the medical device industry in a variety of business development, project management, and operational positions. Most recently, he spearheaded the successful start-up of Machine Solutions, GmbH in Munich, Germany and spent three years building a European operations team as a subsidiary of Machine Solutions Inc before successfully overseeing the transition to an EU based operational team. His experience in the minimally-invasive field covers a wide range of products including DES, Structural Heart Devices, and Peripheral Intervention devices. He received his B.S. in Mechanical Engineering from Cal Poly San Louis Obispo in 2003.
| Leadership Team |
 |
 Mr. Daniel Kasprzyk has over 25 years’ experience in the medical device industry as an executive in startup and multinational business platform companies and holds numerous patents in the device and medical equipment space. He has held Senior Management and Product Development positions in companies such as Medex Inc., Advanced Cardiovascular Systems (Guidant Corporation), Spectranetics Inc., B. Braun Medical, and W.L Gore & Associates. He has co-founded successful medical startup ventures, Vascular Solutions and Machine Solutions, and recently founded Poba Medical. He received his B.S.E. in Bioengineering from Arizona State University.
Mr. Daniel Kasprzyk has over 25 years’ experience in the medical device industry as an executive in startup and multinational business platform companies and holds numerous patents in the device and medical equipment space. He has held Senior Management and Product Development positions in companies such as Medex Inc., Advanced Cardiovascular Systems (Guidant Corporation), Spectranetics Inc., B. Braun Medical, and W.L Gore & Associates. He has co-founded successful medical startup ventures, Vascular Solutions and Machine Solutions, and recently founded Poba Medical. He received his B.S.E. in Bioengineering from Arizona State University.
 Mr. Sohail Desai is Principal Engineering Leader. Mr. Desai is 36 years old with 15 years of Medical Device Industry experience. Prior to joining Symple Surgical he held key roles at major Medical Device Companies, Stryker and AngioDynamics as an Electrical Engineer, Product Development Engineer, and Manufacturing Engineer. His past responsibilities have included product development, product validation, device commercialization, and new product launches. He holds a B.S. in both Biomedical Engineering and Electrical Engineering from Johns Hopkins University.
Mr. Sohail Desai is Principal Engineering Leader. Mr. Desai is 36 years old with 15 years of Medical Device Industry experience. Prior to joining Symple Surgical he held key roles at major Medical Device Companies, Stryker and AngioDynamics as an Electrical Engineer, Product Development Engineer, and Manufacturing Engineer. His past responsibilities have included product development, product validation, device commercialization, and new product launches. He holds a B.S. in both Biomedical Engineering and Electrical Engineering from Johns Hopkins University.
 Mr. Gary Seelhorst is Chief Business Officer. Mr. Seelhorst has over 25 years of medical device and pharmaceutical clinical and corporate development experience with both large-cap pharmaceutical companies like Eli Lilly and Pfizer as well as start-up ventures including extensive capital raising, licensing and M&A transactions as well closing strategic agreements with major PBMs, third party payers, and hospital/surgical centers. He has also worked as a management consultant for several start-ups both raising capital and setting up strategic joint ventures in both the pharmaceutical, medical device, and nanotechnology spaces. Gary served as Vice President of Business Development at Imprimis Pharmaceuticals, Inc. since July 2013 until June 2017. In 2012, he worked for HUYA Biosciences International in Global Business Development setting up both in-licensing and strategic partnerships primarily in China. From 2006 to 2011, he served as Vice President of Business Development & Strategic Marketing for Naviscan, Inc., developing, manufacturing, and selling a mobile PET scanner. Before joining Naviscan, from 1998 to 2006 he worked at Pfizer in various positions in Clinical Development as well as Corporate Development, helping to initiate a burgeoning in-licensing and M&A campaign which resulted in several large M&A transactions. From 1996 to 1998, he worked as a Medical Writer for Eli Lilly with his entire tenure devoted to writing a successfully approved NDA for Prozac. Gary graduated from UC San Diego with a double major in Biochemistry and Psychology, from Indiana University with an MS is Biology, and from University of Michigan with an MBA.
Mr. Gary Seelhorst is Chief Business Officer. Mr. Seelhorst has over 25 years of medical device and pharmaceutical clinical and corporate development experience with both large-cap pharmaceutical companies like Eli Lilly and Pfizer as well as start-up ventures including extensive capital raising, licensing and M&A transactions as well closing strategic agreements with major PBMs, third party payers, and hospital/surgical centers. He has also worked as a management consultant for several start-ups both raising capital and setting up strategic joint ventures in both the pharmaceutical, medical device, and nanotechnology spaces. Gary served as Vice President of Business Development at Imprimis Pharmaceuticals, Inc. since July 2013 until June 2017. In 2012, he worked for HUYA Biosciences International in Global Business Development setting up both in-licensing and strategic partnerships primarily in China. From 2006 to 2011, he served as Vice President of Business Development & Strategic Marketing for Naviscan, Inc., developing, manufacturing, and selling a mobile PET scanner. Before joining Naviscan, from 1998 to 2006 he worked at Pfizer in various positions in Clinical Development as well as Corporate Development, helping to initiate a burgeoning in-licensing and M&A campaign which resulted in several large M&A transactions. From 1996 to 1998, he worked as a Medical Writer for Eli Lilly with his entire tenure devoted to writing a successfully approved NDA for Prozac. Gary graduated from UC San Diego with a double major in Biochemistry and Psychology, from Indiana University with an MS is Biology, and from University of Michigan with an MBA.
| Our Core |
 |
 Symple Surgical has a dedicated team of experienced Medical Device professionals and world-class contract suppliers committed to developing and producing the most advanced technology for the market. We utilize expert consultants in specialized fields and maintain a core team exclusively focused to the development and production of our proprietary technology. As an early-stage start-up we are committed to running a lean operation.
Symple Surgical has a dedicated team of experienced Medical Device professionals and world-class contract suppliers committed to developing and producing the most advanced technology for the market. We utilize expert consultants in specialized fields and maintain a core team exclusively focused to the development and production of our proprietary technology. As an early-stage start-up we are committed to running a lean operation.
| Scientific Advisory Board |
 |
 Dr. Nicholas Shaheen
Dr. Nicholas Shaheen
Dr. Shaheen is Professor of Medicine and Epidemiology and Chief of the Division of Gastroenterology and Hepatology at the UNC School of Medicine and UNC School of Public Health, and Director of the UNC Center for Esophageal Diseases and Swallowing. He is a leading researcher in the field of Ablative Therapies for the treatment of Barrett’s Esophagus and a Primary Investigator for Clinical Trials for BarrX and CSA Medical.
 Dr. Prasad Iyer
Dr. Prasad Iyer
Professor of Medicine, Mayo Clinic Center for Clinical and Translational Science, Rochester, MN
Consultant, Division of Gastroenterology and Hepatology
Co-Director, Advanced Esophageal Fellowship
 Dr. Christopher Brace PhD
Dr. Christopher Brace PhD
Professor, Departments of Radiology and Biomedical Engineering, Director of the Tumor Ablation Laboratory, University of Wisconsin School of Medicine. Leading Researcher on Microwave Ablation
 Dr. Gary Ansel
Dr. Gary Ansel
Board Certified in Internal Medicine with a subspecialty in Cardiovascular Disease, Clinical Director of Peripheral Vascular Interventional Medicine, Riverside Methodist Hospital, Columbus Ohio; Board Member VIVA Conference; Co-Founder and Chief Medical Officer, Nexeon MedSystems Inc.; Ostial Solutions LLC.
 Mr. Bob Haggerty
Mr. Bob Haggerty
Former Vice President of Sales & Marketing at BARRX Medical prior to and after the acquisition by Medtronic/Covidien. B.S. Business Administration, Wake Forest University. Actively involved with startup financing and multiple startup exits including Ceterix Orthopaedics, ReShape Medical, BARRX Medical, ACMI, and Boston Scientific Corporation.

|
|
Resources |
 |
| Barrett’s Esophagus and Esophageal Adenocarcinoma |
 |

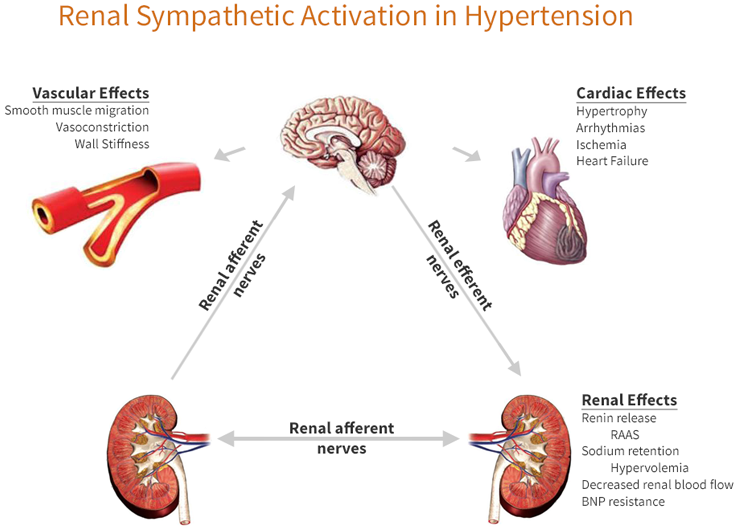
| Hypertension and Resistant Hypertension |
 |
- Heart disease caused by hypertension, is the leading causes of death in U.S and globally
- Stroke is the fifth leading causes of death domestically and worldwide
- Hypertension accelerates loss of kidney function in over 500m people worldwide and 26m Americans
- The global incidence of Hypertension among adults is expected to increase 60% by 2025
| Why Microwave |
 |
| Microwave vs. RF Heating |
 |
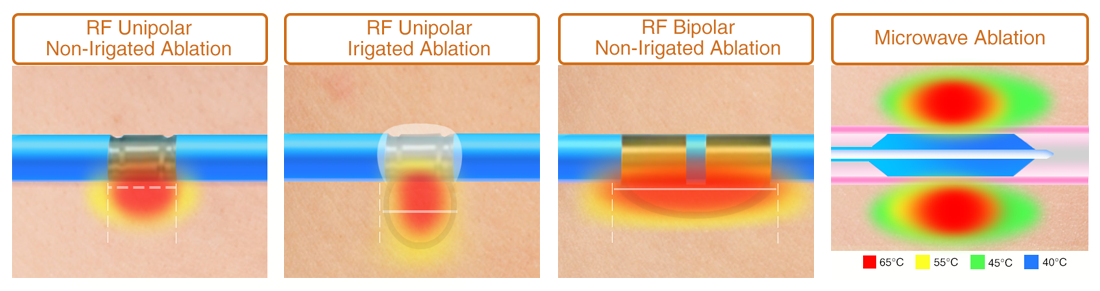 RF Ablation has limited capability compared to Microwave Ablation. Advantages of Microwave Ablation include:
RF Ablation has limited capability compared to Microwave Ablation. Advantages of Microwave Ablation include:
- Faster Heating
- Larger Heating Zone
- More Uniform Heating Zone
- Less dependent on Tissue Properties
- No grounding pad or 2nd pole necessary
- Tissue contact is not necessary
- Independent of wall pressure
| Reimbursement Landscape |
 |
| Beyond Barrett’s |
 |

|
|
Information Center |
 |
| Follow SSI |
 |
| News Feed |
 |
-
Articles, 2018
- Barrett's esophagus: Treatment with radiofrequency ablation - UpToDate
- PENTAX Medical Introduces Next-Generation C2 CryoBalloon™ Ablation System for Treatment of Barrett's Esophagus
- Global Barrett’s Esophagus Ablation Device Market 2018 Assessment - Brainlab, Medtronic and GE Healthcare
- Study tracks evolutionary transition to destructive cancer
- Swallowable Balloon Device Detects Barrett's Esophagus
- New York Times, 9/11/2015
- Science Daily, 4/21/2015
- American Heart Association, 3/31/15
- Time Magazine, 3/26/15
- Forbes, 9/12/12
| Press Releases |
 |
| Careers |
 |
Symple Surgical is a dynamic start-up medical device company working on ground-breaking approaches to treat enormous global health problems. We offer an exciting and flexible work environment with unique benefits to motivated individuals looking to be part of an energetic team in a challenging field.
Current Open Positions:
- Engineer II
- Senior Catheter Engineer
- Engineering Technician
- Vice President of Clinical and Regulatory Affairs
If you would like to apply for the available position, please enter your contact information in the Contact box below.
Please check back regularly for updated Job Postings.
| Investors |
 |
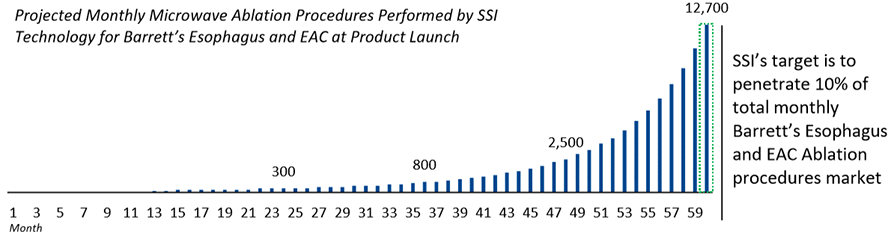
Symple Surgical, Inc. (the “Company” or “SSI”) was established in 2012 with a vision of providing low-cost, minimally invasive, microwave ablation (“MWA”) technology. In 2016, SSI began its focus on treating Barrett’s Esophagus and Esophageal Adenocarcinoma (“EAC”) due to the potential advantages of MWA over the current standard of care. EAC, as a disease, is the fastest growing solid tumor cancer in the world, and SSI has developed a novel method of ablation using microwave frequencies to safely and effectively ablate Barrett’s Esophagus and subsequently eliminate EAC. This disease state has grown by over 800% over the last 30 years and represents a large market opportunity for new treatment methods.
The Company’s founder, Dan Kasprzyk, is a medical device engineer and serial entrepreneur with a track record of successful start-ups and exits. He has primarily funded the Company with approximately $2.0 million, along with placing relevant intellectual property (IP) into the firm. The Company’s prior rounds of financing have raised approximately $1.7 million in funding beyond that invested by Mr. Kasprzyk. The Company now seeks investors to fund the remaining capital needs for the next significant milestone, proof of concept. This includes prototyping of the GRIZZLY™ catheter, handle automation, antennae prototyping, and pre-clinical studies.
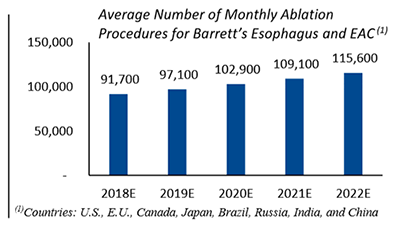
SSI’s microwave ablation method is superior to other ablation methods for treating Barrett’s Esophagus and EAC and provides the following:
- Larger ablation zone killing commonly missed deeper cells
- Microwave energy delivery that is independent of target tissue properties and is non-contact
- Dielectric heating creating a uniform heating method
- Potential for fewer follow-up procedures and less pain
- Ability for circumferential and directional ablations from a single device
- Microwave antennae for monitoring and emission of energy
To date, the Company’s engineering and Scientific Advisors have contributed initial concepts and designs to the Company. Intellectual property reviews were performed prior to forming the Company and eight (8) patent applications associated with the various alternatives have been submitted.
The Company has established initial development plans and timelines for the completion of prototypes, assessment of the alternatives, and starting both animal and FIM studies. SSI’s Management team has significant experience in completing the 510(K) process and successfully commercializing medical products. Below outlines Management’s product rollout plan with an initial focus on outpatient facilities: